 11 October 2017
Have astronomers found the missing mass in the Universe?
11 October 2017
Have astronomers found the missing mass in the Universe?
... ago, astronomers thought that the Universe was composed almost entirely of "baryonic matter" – ordinary atoms made up of protons, neutrons and electrons. But, after calculating the density and composition of the Universe from measurements of the...
 16 October 2017
First detected neutron star merger reveals more than gravitational waves
16 October 2017
First detected neutron star merger reveals more than gravitational waves
... heavy elements like gold and platinum. Again, the previous culprit to explain the formation of atoms with a proton count greater than 26 fell to that of a supernova, as the energy and amount of neutrons released as the core collapses...
 06 December 2017
The next generation planet hunter is here!
06 December 2017
The next generation planet hunter is here!
...hoping to acquire the most accurate measurements of the fundamental constants α (the fine structure constant) and μ (the proton to electron mass ratio) as a function of redshift. Such tiny changes are predicted by some theories of fundamental physics...
 23 July 2018
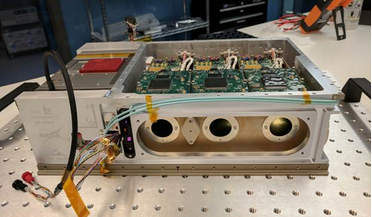
Small box with big designs to find the missing matter in the Universe
23 July 2018
Small box with big designs to find the missing matter in the Universe
... space from around 400,000 after the big bang, scientists have calculated that ‘normal’ matter – protons, neutrons and other subatomic particles that make up all of the stuff we see all around us such as planets...
 09 November 2018
Leftover gas from the Sun's birth created Earth's water
09 November 2018
Leftover gas from the Sun's birth created Earth's water
... all hydrogen atoms are the same. One type is ‘heavier,’ as along with the standard electron and proton that a normal hydrogen atom contains, it packs in an extra neutron in its nucleus. This heavy hydrogen is called deuterium...
 22 May 2019
Water came to Earth when the Moon formed says study
22 May 2019
Water came to Earth when the Moon formed says study
... is essential for life; molybdenum. An isotope is a variant of a particular chemical element which has the same number of protons, but a different number of neutrons in each atom. Molybdenum does not occur naturally as a free metal on Earth because...